
GORE® SYNECOR Biomaterial
2.5x stronger than BD® BARD® Soft Mesh*,3,4
14% stronger than BD® VENTRALIGHT® ST Mesh*,4,5
ZERO fractures reported†,‡
Permanent mesh solution for ventral hernia repair with the strength of a heavyweight, built like a lightweight and a low rate of infection for high BMI or co-morbid patients.1-7 All these benefits are realized at a lower cost than BD® VENTRALIGHT® ST Mesh.†,§
STRENGTH
Permanent devices
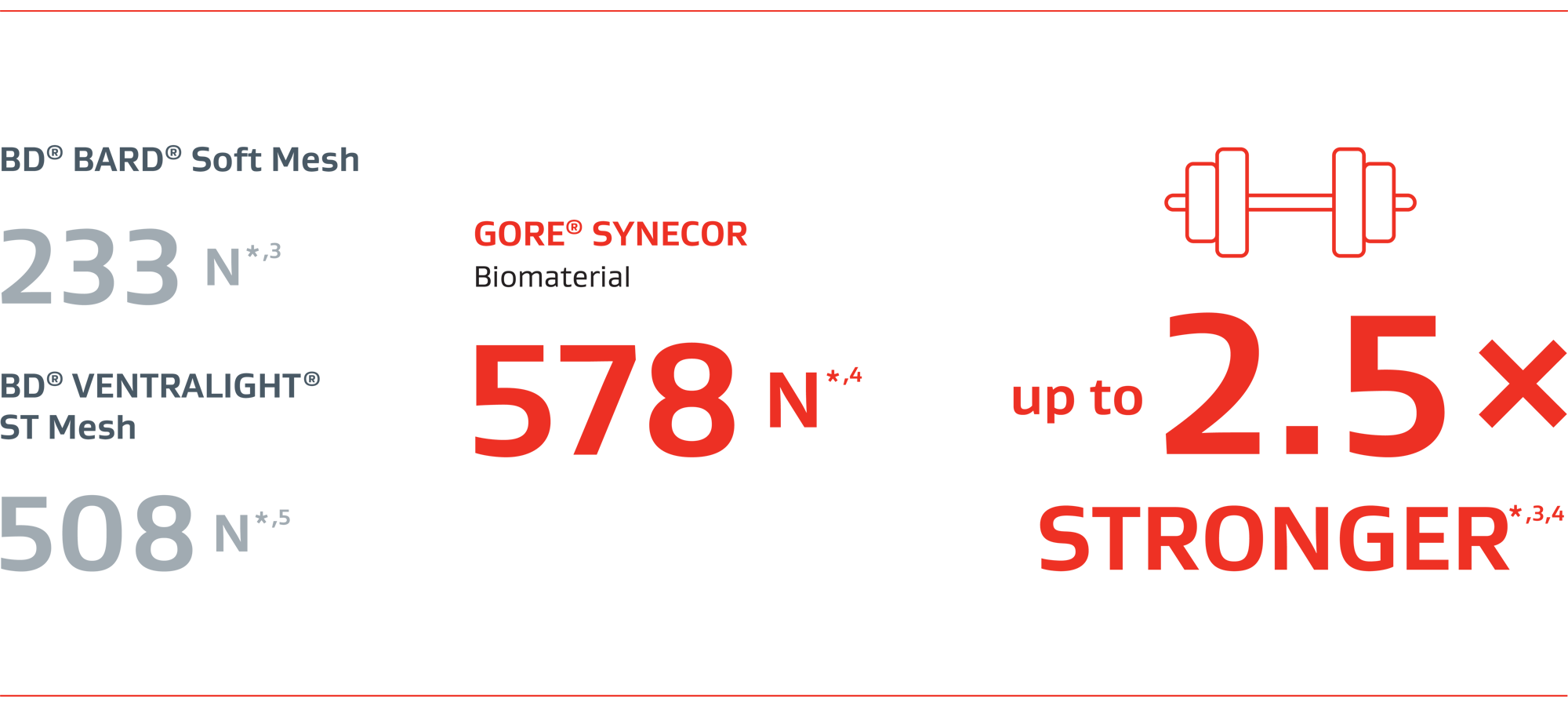
Ball burst strengthII (N)
GORE® SYNECOR Biomaterial

< 1% recurrence rate in ventral hernia working group (VHWG) 1 and 2 patients1,2
< 2.2% surgical site infection (SSI) rate over 12-24 months1,2

Easy to use in minimally invasive procedures (laparoscopic, robotic) and open surgeries6,7
See the real-world evidence:
Evaluation of long-term performance of an intraperitoneal biomaterial in the treatment of ventral hernias—Surgical Endoscopy December, 2023

Gore ventral hernia solutions balance strength with a low rate of complications for your high-risk patients which is backed by†,¶:
- 15 years of extensive clinical literature in soft tissue reinforcement†
- Over 200 publications††
- More than 5,000 cases in literature††
Largest range of sizes‡‡: now you can choose just the right size.
Up to 40 cm x 50 cm. You now have more choices to get the right fit for your patients.
Is an absorbable device what your co-morbid patients need?
GORE® ENFORM Biomaterial is 25% stronger than BD® PHASIX® Mesh with zero mesh removals or erosions reported.†, **, ††,§§,8
See it in action.
* Includes only the permanent component of these products.
† Data on file, W. L. Gore & Associates, Inc; Flagstaff, AZ.
‡ No confirmed reports of fracture.
§ Cost savings are based on 90 days post-operative outcomes.
II Bench-top evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results.
¶ GORE® BIO-A® Tissue Reinforcement, GORE® ENFORM Biomaterial and GORE® SYNECOR Biomaterial.
** No confirmed or published complete mesh removals due to infection or erosions reported.
†† A literature search was performed by an Information Specialist in 2024 using the DIMENSIONS® Database, EMBASE® Database, and MEDLINE® on DIALOG® Database. Key words/phrases are on file.
‡‡ Largest range of sizes currently available in a permanent heavyweight soft tissue reinforcement device in the United States as of July 2024.
§§ Out-of-the-box strength.
- Linn JG, Mallico EJ, Doerhoff CR, Grantham DW, Washington RG Jr. Evaluation of long-term performance of an intraperitoneal biomaterial in the treatment of ventral hernias. Surgical Endoscopy 2023;37:3455-3462.
- Rios-Diaz A, Hitchner M, Christopher AN, Broach R, Cunning JR, Fischer JP. Early clinical and patient-reported outcomes of a new hybrid mesh for incisional hernia repair. Journal of Surgical Research 2021;265:49-59.
- Olson TB. Competitive Hernia Device Strength Evaluation. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108484.
- W. L. Gore & Associates, Inc. Plexus Knit PQ Validation Report. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. [Validation Report]. MD145325. Rev 5.
- Olson TB. Ventralight ST Strength after 14 and 28 day Degradation of Absorbable Components. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108781.
- W. L. Gore & Associates, Inc. GORE SYNECOR Intraperitoneal Biomaterial Design Control (DC) Matrix. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2023. [Design Control Matrix – DC Matrix]. MD184278. Rev 3.
- W. L. Gore & Associates, Inc. GORE SYNECOR Preperitoneal Biomaterial Design Control (DC) Matrix. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2023. [Design Control Matrix – DC Matrix]. MD187124. Rev 3.
- Deeken CR, Matthews BD. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-PHASIX Mesh) in a porcine model of hernia repair. ISRN Surgery 2013;2013:238067.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
BD, BARD, PHASIX and VENTRALIGHT are trademarks of C.R. Bard, Inc.
DIMENSIONS is a trademark of Digital Science & Research Solutions Inc.
EMBASE is a trademark of Elsevier Limited.
DIALOG is a trademark of Proquest LLC.
MEDLINE is a trademark of The National Library of Medicine.
24GP1030-EN01

